Chance News 99: Difference between revisions
(Created page with "==Quotations== ==Forsooth== ==Item 1== ==Item 2==") |
(→Item 1) |
||
| Line 3: | Line 3: | ||
==Forsooth== | ==Forsooth== | ||
== | ==Renal denervation== | ||
[http://www.nytimes.com/2014/03/30/health/setback-for-promising-high-blood-pressure-treatment.html?hp&_r=0 Setback for high blood pressure treatment]<br> | |||
by Denise Grady, ''New York Times'', 29 march 2014 | |||
According to the article, | |||
"In the United States, 67 million people have high blood pressure, and it resists treatment [by drugs] in about 10 percent of them." | |||
Thus, the interest in | |||
<blockquote> | |||
The treatment, called renal denervation, [which] involves threading a tube through blood vessels into the renal arteries, [and] then zapping them with radio-frequency energy to kill nerve endings. | |||
<br><br> | |||
The procedure was thought to be a lifesaver for people whose high blood pressure could not be lowered even with multiple drugs. Uncontrolled hypertension increases the risk of strokes, heart attacks and other problems. | |||
</blockquote> | |||
Previous “case reports and studies had found astounding drops in blood pressure after the [renal denervation] treatment — as much as 30 millimeters of mercury in systolic pressure, the top number in a blood pressure reading.” Therefore, to the surprise of many, renal denervation [http://www.nejm.org/doi/pdf/10.1056/NEJMoa1402670 failed in a large, rigorous study]. | |||
Unlike earlier, unblinded studies, this so-called SYMPLICITY HTN-3 study was | |||
<blockquote> | |||
a prospective, single-blind, randomized, sham-controlled trial. Patients | |||
with severe resistant hypertension were randomly assigned in a 2:1 ratio to undergo renal denervation or a sham procedure. | |||
<br><br> | |||
The primary efficacy end point was the change in office systolic blood pressure at 6 months; a secondary efficacy end point was the change in mean 24-hour ambulatory systolic blood pressure. | |||
</blockquote> | |||
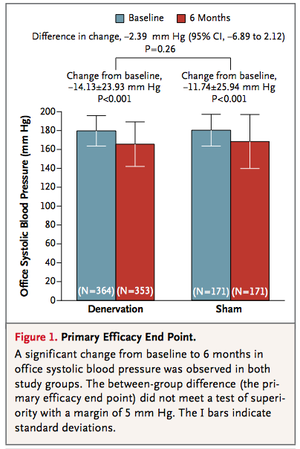
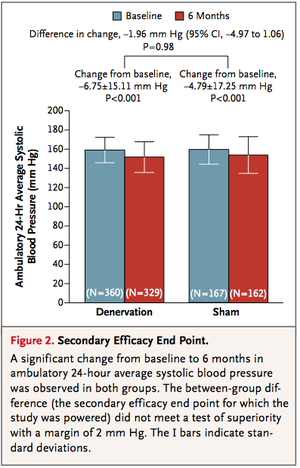
From the figures below, it can be seen that the treatment (renal denervation) and the control (sham treatment) are virtually identical for the primary and secondary efficacy, respectively. That is, for primary efficacy, the treatment failed to beat the control by at least 5 mm HG and for secondary efficacy, the treatment failed to beat the control by at least 2 mm Hg. | |||
<center> | |||
[[File:Renal_Denervation1.png | 300px]] | |||
[[File:Renal_Denervation2.png | 300px]] | |||
</center> | |||
===Discussion=== | |||
1. Franz H. Messerli [http://www.nejm.org/doi/full/10.1056/NEJMe1402388?query=featured_home wrote an editorial in the NEJM] entitled “Renal Denervation for Resistant Hypertension?” in which he questioned the enthusiasm for the procedure. It has not been approved in the United States but according to Grady,”it has been approved in more than 80 countries and performed on thousands of patients.” She quotes Messerli as saying | |||
<blockquote> | |||
You have to perhaps congratulate the Food and Drug Administration that they were not as eager to approve this procedure based on the little evidence there was, as opposed to the Europeans and the Australians. | |||
<br><br> | |||
It [SYMPLICITY HTN-3] is absolutely a landmark study. I hope it will have an impact and will override some of the zeal of the European investigators. | |||
</blockquote> | |||
2. As indicated above, the (15) authors of the study regard “clinical (i.e., practical) significance” to be a primary superiority of at least 5 mm Hg or a secondary superiority of at least 2 mm Hg of the treatment over the control. Neither was achieved but with the help of any convenient statistics software, use the numbers in Figure 1 and Figure 2 to show that “statistical” significance was also not achieved in either case for the respective differences between treatment and control. | |||
3. The study also looked at subgroups such as race, gender, age, etc. | |||
<blockquote> | |||
Although the differences between groups in some subgroups were nominally significant, the absolute magnitude of the differences was small (<10 mm Hg), and the differences were not significant with the use of a superiority margin of 5 mm Hg or after adjustment for multiple comparisons. | |||
</blockquote> | |||
Which “significances” are being discussed here? | |||
4. Point out the similarities of a sham treatment to the use of a placebo when testing drugs. Point out the differences between a sham treatment and a placebo. | |||
Submitted by Paul Alper | |||
==Item 2== | ==Item 2== | ||
Revision as of 02:16, 8 April 2014
Quotations
Forsooth
Renal denervation
Setback for high blood pressure treatment
by Denise Grady, New York Times, 29 march 2014
According to the article, "In the United States, 67 million people have high blood pressure, and it resists treatment [by drugs] in about 10 percent of them."
Thus, the interest in
The treatment, called renal denervation, [which] involves threading a tube through blood vessels into the renal arteries, [and] then zapping them with radio-frequency energy to kill nerve endings.
The procedure was thought to be a lifesaver for people whose high blood pressure could not be lowered even with multiple drugs. Uncontrolled hypertension increases the risk of strokes, heart attacks and other problems.
Previous “case reports and studies had found astounding drops in blood pressure after the [renal denervation] treatment — as much as 30 millimeters of mercury in systolic pressure, the top number in a blood pressure reading.” Therefore, to the surprise of many, renal denervation failed in a large, rigorous study.
Unlike earlier, unblinded studies, this so-called SYMPLICITY HTN-3 study was
a prospective, single-blind, randomized, sham-controlled trial. Patients with severe resistant hypertension were randomly assigned in a 2:1 ratio to undergo renal denervation or a sham procedure.
The primary efficacy end point was the change in office systolic blood pressure at 6 months; a secondary efficacy end point was the change in mean 24-hour ambulatory systolic blood pressure.
From the figures below, it can be seen that the treatment (renal denervation) and the control (sham treatment) are virtually identical for the primary and secondary efficacy, respectively. That is, for primary efficacy, the treatment failed to beat the control by at least 5 mm HG and for secondary efficacy, the treatment failed to beat the control by at least 2 mm Hg.
Discussion
1. Franz H. Messerli wrote an editorial in the NEJM entitled “Renal Denervation for Resistant Hypertension?” in which he questioned the enthusiasm for the procedure. It has not been approved in the United States but according to Grady,”it has been approved in more than 80 countries and performed on thousands of patients.” She quotes Messerli as saying
You have to perhaps congratulate the Food and Drug Administration that they were not as eager to approve this procedure based on the little evidence there was, as opposed to the Europeans and the Australians.
It [SYMPLICITY HTN-3] is absolutely a landmark study. I hope it will have an impact and will override some of the zeal of the European investigators.
2. As indicated above, the (15) authors of the study regard “clinical (i.e., practical) significance” to be a primary superiority of at least 5 mm Hg or a secondary superiority of at least 2 mm Hg of the treatment over the control. Neither was achieved but with the help of any convenient statistics software, use the numbers in Figure 1 and Figure 2 to show that “statistical” significance was also not achieved in either case for the respective differences between treatment and control.
3. The study also looked at subgroups such as race, gender, age, etc.
Although the differences between groups in some subgroups were nominally significant, the absolute magnitude of the differences was small (<10 mm Hg), and the differences were not significant with the use of a superiority margin of 5 mm Hg or after adjustment for multiple comparisons.
Which “significances” are being discussed here?
4. Point out the similarities of a sham treatment to the use of a placebo when testing drugs. Point out the differences between a sham treatment and a placebo.
Submitted by Paul Alper